Coordinator’s Message
Dear visitors,
MIMVaC-Africa is an ambitious programme that aims to test a portfolio of five malaria vaccine candidates to identify the most promising candidate that could be tested in large Phase 3 trials. In addition, the programme will help strengthen clinical trial infrastructure and vaccine development capabilities at African sites.
The multilateral collaboration of experts from ten Asian, European and African institutions; convergence of resources and energies; and the use of modern research techniques such as controlled malaria infections in humans, are assets that will enable our consortium to achieve its objectives on time.
Since the official launch of the program on 01 February 2020, I am very pleased to see activities gradually being deployed on the ground despite the Covid-19 pandemic constraints. This is an opportunity for me to express my gratitude to the partner institutions and to the European & Developing Countries Clinical Trials Partnership (EDCTP) of the European Union, which is funding our initiative to the tune of more than 11 million Euros.
I look forward to the development of malaria vaccine, the ultimate response to save thousands of young lives lost early due to malaria.
Dr Sodiomon B. SIRIMA
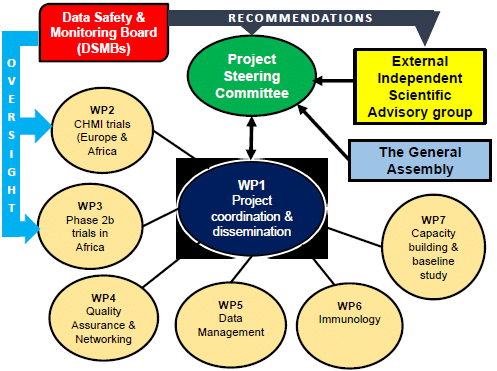
Organizational structure
An independent ethics advisor (EA) is appointed. The ethics advisor is an independent expert in bioethics free of any conflict of interests. The EA maintains an overview of operations throughout the project implementation, helping with preparation in terms of thinking ahead about possible problems and how they can be addressed. He/she provides advice and guidance any time an ethical dilemma arise and ensure consortium research activities are in compliance with ethical standards within the research field of malaria vaccine development. The EA is fully integrated into the management structure, and may attend plenary meetings, participate in all significant technical group meetings, review all annual reports and papers for publication and help in monitoring all the authorizations, approvals and licences. His/her inputs and advice is minuted and reports are prepared.
The General assembly (GA) of MIMVaC-Africa includes one representative from each consortium member and meets once a year. The GA has not any role in decision making. This is the larger group which include other site staff who are not member of the PSC but play a significant role in project execution on the sites. The annual meeting of the assembly presents progress, discuss issues then the PSC in a close meeting review issues and make decision.
Independent trial specific Data and Safety Monitoring Boards (DSMBs) is established for the SPICy, CRISPy and VALiT trials. The role of the DSMBs will be to review study conduct and progress as well the data on the safety and efficacy and provide recommendations to both the PSC and the EISAG about the continuation, modification or termination of any trial. The DSMBs operates under its own charter.
PSC (Project Steering Committee)
The Project Steering Committee (PSC) is chaired by the project coordinator for the entire project duration and a vice chair elected for a one year mandate not renewable. The PSC includes the leaders and co-leaders of the work package plus one representative from each academic/industry partner if there are not leading any Work Package. Procedures of decision-making are defined in the consortium agreement. As a general rule, decisions shall be taken by a majority of two-thirds (2/3) of the votes cast. The PSC meets at least quarterly and will have conference calls and continuous contact by e-mail and other means as needed, to monitor and guide the activities of the consortium towards achieving the objectives defined.
EISAG (External Independent Scientific Advisory Group)
For specific advisory activities an External Independent Scientific Advisory Group (EISAG) is formed. The EISAG counsels the consortium on scientific matters. Their contribution is minuted. The EISAG constitutes by three (3) to maximum five (5) experts from different fields (malaria, vaccinology, regulatory, biostatistics, ethicists) who are appointed to advise on request the project consortium to achieve its goals. The EISAG reviews the overall proposed studies and activities within the project and provide recommendations to the PSC prior to the project start. The EISAG is informed on the project conduct on a regular basis and is consulted at each of the critical phase of the project where decision to progress from one phase to another is to be made. A representative from EDCTP acts as an observer on this committee.
The External Independent Scientific Advisory Group (EISAG) is constituted by experts from different fields (malaria, vaccinology, regulatory, biostatistics, ethicists) who are appointed to advise on request the project consortium to achieve its goals. The EISAG reviews the overall proposed studies and activities within the project and provide recommendations to the PSC prior to the project start. The EISAG is kept informed on the project conduct on a regular basis and is provided with the CRISPY study outcomes to make an independent decision of the candidates’ vaccine that will be taken forward to the phase 2b study.